Creating a Data Source
To create a new data source, you can go to the Data Sources tab under Manage Study, then create a data source from the following types:
Surveys/Forms - Learn about building surveys here: Building: Surveys
Devices - For physical devices
SMS/Other - For data sources that aren’t surveys or physical devices
Clarity - Custom Data Source (via API)
Clinstream
PennchartX ADT feeds
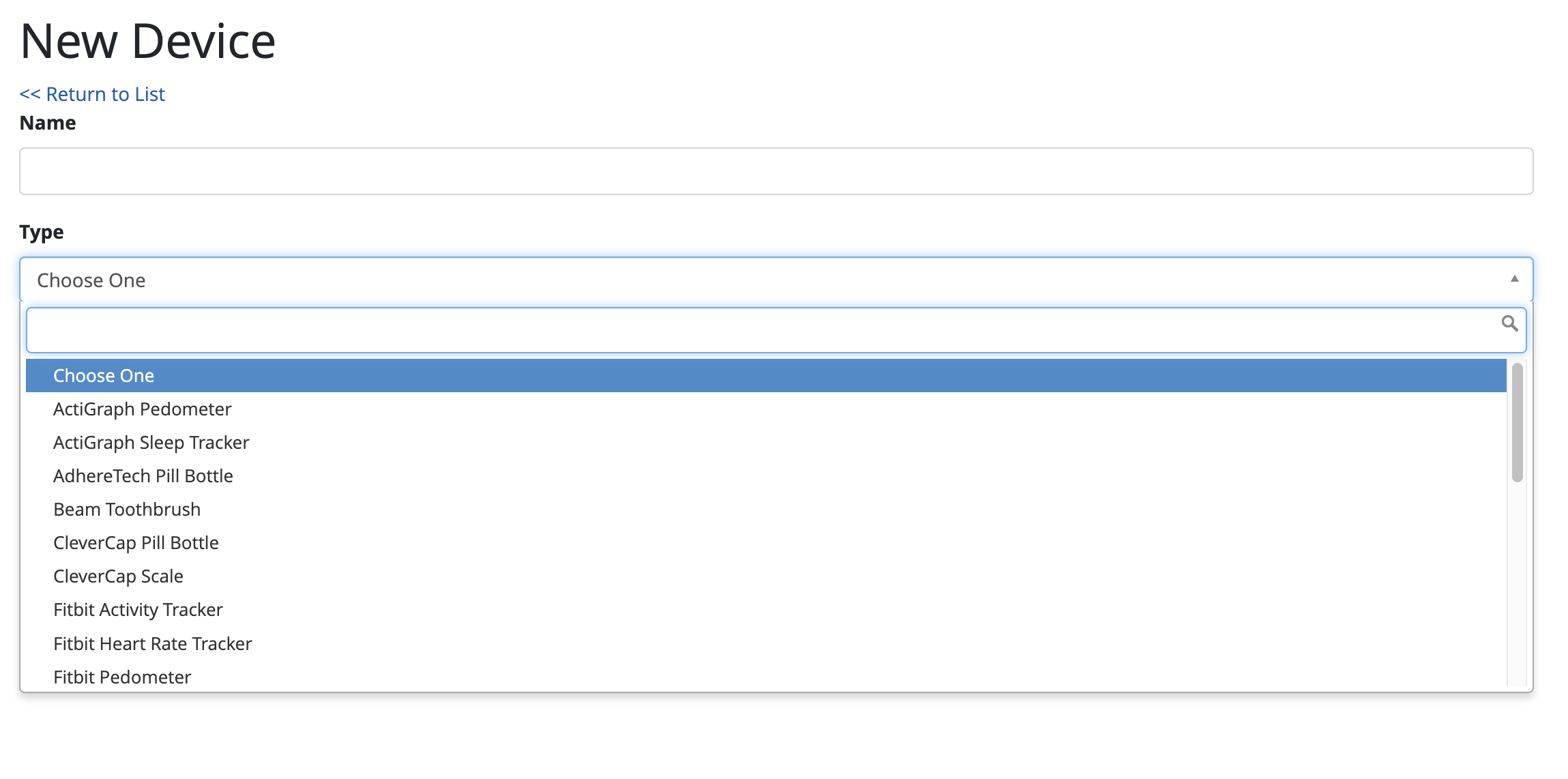
Adding a New Device
For a list of our supported devices, look here: https://waytohealth.atlassian.net/wiki/spaces/BG/pages/2567110660/Supported+data+sources#%5BhardBreak%5DConnected-Devices
The first step to setting up a device integration is to select which device you are trying to collect data from:
Once a device has been selected, you will be presented with any additional pieces of information that may be required for Way to Health to interact with that device vendor. This is usually either a key provided by them for authentication or a username/password.
About Device Authorization
Some devices are authorized by the participant during enrollment (e.g. Fitbit and Withings) while others are authorized at the study level (e.g. Adheretech). For devices that are authorized by the participant during enrollment, you will want to add a Device Authorization Enrollment Step to the study configuration.
About Data Collection
When it comes to physical devices, some of them are polled every hour for new data, while others are configured to have new data pushed to Way to Health. You can read more about these differences here [tbd].
Advanced Settings
There are advanced settings within each device that can impact how data is processed for the device on a more detailed level. Most devices have default values for these settings but there may be situations where you want to change them.
Collect Time Series Data enables intraday data collect for devices that support it.
Selection Strategy explanations can be found in How Device Data attaches to Events. However there are some general principles..
First: used for medication adherence devices
Last:
Min: used for weight loss studies
Max: used for physical activity studies
Selection Field determines which field of data WTH should use for a particular device to determine whether a participant completed or was compliant to the encounter
Ex: Clevercap pill bottle selection field should be IS_TAKEN, which will have the variables 0 and 1 for yes and no to determine encounter completeness and compliance
Attach data and close encounter... indicates when the encounter should close.
As soon data is available: will immediately close out the encounter once data is received and attaches to the open encounter.
Generally used for medication adherence devices
Wait until the end of the encounter window: encounter will not close until the end of the encounter window. This is ideal for devices where it may be typical to receive multiple data enteries throughout the course of the encounter.
Ex: leave Fitbit encounter window open so that WTH attaches the maximum number of steps walked to the encounter.
Ex: leave Withings Scale encounter window open so that WTH attaches the minimum weight value to the encounter, regardless of how many times the participant steps on the scale.
When an already-completed encounter gets new data... determines what to do with new data
Attach new data in place of the old: If new data comes in after an encounter is completed, it will switch out the new data with original data
Ex: Fitbit participant doesn't sync their device until the following day, WTH will replace the '0 steps' with the # of steps that they walked
Stick with our original data: WTH should leave the original data that attached to the encounter
Used for medication adherence devices
Apply feedback... indicates what action WTH should take following new data
Only the first time data attaches: If any new data comes in during an encounter window that has already been completed, WTH will not do anything.
Each time a new data point attaches to an encounter: If any new data comes in during an encounter window that has already been completed, WTH will reapply feedback on the encounter for each data entry
If data attaches late to an event... allows for creation of an incident in the case of late data.